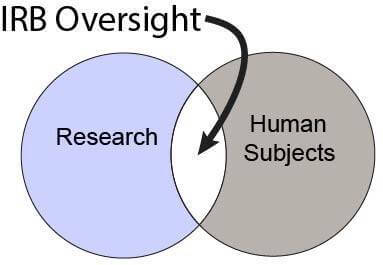
Is IRB Approval Required?

| HHS Definition of Research (Common Rule) (45 CFR 46.102(l))“A systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.”In general activities that contribute to generalizable knowledge are those that:attempt to make comparisons or draw conclusions based on the data;seek underlying principles that have predictive value and can be applied to other circumstances;identify general explanations or themes that a reader can extrapolate to another situation. Although publication is often viewed as evidence of research status, it is not the only criterion. In fact, “systematic investigations” often result in published information, yet they do not qualify as research because they were not designed to contribute to generalizable knowledge. |
| HHS Definition of a Human Subject (Common Rule) (45 CFR 46.102(e)) Human subject – A living individual about whom an investigator (whether professional or student) conducting research:(1) Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens;or(2) Obtains, uses, studies, analyzes, or generates identifiable private information or biospecimens. Intervention includes both physical procedures by which data are gathered (e.g. venipuncture) and manipulations of the subject or the subject’s environment that are performed for research purposes. Interaction includes communication or interpersonal contact between investigator and subject. Private information includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, as well as information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (e.g. a medical record). Identifiable information means that the identity of the subject is, or may be readily ascertained (directly or indirectly) by the investigator (or others) or associated with the information. |
| FDA Definition of a Clinical Investigation (21 CFR 50.3(c))“Any experiment that involves a test article and one or more human subjects that is either subject to requirements for prior submission to the Food and Drug Administration under section 505(i), or 520(g) of the act, or is not subject to requirements for prior submission to the Food and Drug Administration under these sections of the act, but the results of which are intended to be submitted to, or held for inspection by the Food and Drug Administration as part of an application for a research or marketing permit.” The term does not include experiments that are subject to the provision of 21 CFR 58, regarding nonclinical laboratory studies.Under FDA regulations, the terms “research” and “clinical investigation” are synonymous. A test article means any drug (including a biological product for human use), medical device for human use, human food additive, color additive, electronic product, or any other article subject to regulation under the Federal Food, Drug and Cosmetic Act (21 CFR 50.3(j)).FDA regulations generally require IRB review and approval of research involving FDA-regulated products (e.g., investigational drugs, biological products, medical devices and dietary supplements) (21 CFR Part 56). |
| FDA Definition of a Human Subject (21 CFR 50.3(g))FDA Human subject – an individual who is or becomes a participant in research, either as a recipient of the test article or as a control. A subject may be either a healthy human or a patient. |